Fine, the title is an exaggeration. But only a small one. GLP-1 receptor agonist medications like Ozempic are already FDA-approved to treat diabetes and obesity. But an increasing body of research finds they’re also effective against stroke, heart disease, kidney disease, Parkinson’s, Alzheimer’s, alcoholism, and drug addiction.
There’s a pattern in fake scammy alternative medicine. People get excited about some new herb. They invent a laundry list of effects: it improves heart health, softens menopause, increases energy, deepens sleep, clears up your skin. This is how you know it’s a fraud. Real medicine works by mimicking natural biochemical signals. Why would you have a signal for “have low energy, bad sleep, nasty menopause, poor heart health, and ugly skin”? Why would all the herb’s side effects be other good things? Real medications usually shift a system along a tradeoff curve; if they hit more than one system, the extras usually just produce side effects. If you’re lucky, you can pick out a subset of patients for whom the intended effect is more beneficial than the side effects are bad. That’s how real medicine works.
But GLP-1 drugs are starting to feel more like the magic herb. Why?
Medicine is bad at answering “why” questions. Often the answer looks like “because it modulates ABC transmission, which inhibits XYZ, which signals to MNO, and MNO is involved in the disease.” This is all very scientific-sounding but totally fails to satisfy any normal human curiosity.
I’m going to discuss a few of GLP-1 drugs’ effects on this level, but also try to speculate about some broader principles behind why these medicines seem so magical1.
Diabetes
This is the one we understand best.
When a recently-eaten meal reaches food-detector cells in your intestine, they release glucagon-like-peptide 1 (GLP-1). This is one of the many hormones that tell your body that you’ve eaten and that it should adjust its activities accordingly. Various organs have GLP-1 receptors. When they sense GLP-1, they start various digestion-related tasks.
Since eating a meal increases blood sugar, your body wants to push blood sugar back down. So GLP-1 tells the pancreas to release more insulin (sugar ↓) and less glucagon (sugar ↑).
Diabetes involves excessive blood sugar, so this is the profile you want for an antidiabetic drug. But natural GLP-1 decays within a minute or two, so there’s no way to use it as a medication.
In 1992, scientists discovered a chemical in Gila monster venom which looked like GLP-1, activated GLP-1 receptors, but lasted a whole two hours. This became exenatide, the first GLP-1 receptor agonist (one of my favorite paper names is Exenatide: From The Gila Monster To The Pharmacy). By playing around with its structure, Big Pharma was eventually able to create liraglutide (twelve hours), semaglutide (one week), and cafraglutide (one month).
All of these work the same way: they mimic the natural hormone that the intestine produces to tell your body that it’s just eaten.
Weight Loss
Like most good pharmacologic discoveries, the role of GLP-1 drugs for weight loss was partly accidental: we noticed patients losing weight before we understood why. The science has only recently caught up.
There are two plausible places GLP-1 drugs could lower weight: the body or the brain. In the body, they could change stomach contraction rate, hormone production, etc. In the brain, they could control the mental sensation of hunger. To separate these two effects, scientists bred rats that only had GLP-1 receptors in one place or the other. The results were unequivocal: Ozempic and its relatives work in the brain. Although they have some effects in the body, these are short-lived and not really relevant to their mechanism of action for weight loss.
This is pretty surprising: the brain is protected by the blood-brain barrier which usually blocks large molecules. Ozempic is a large molecule. Scientists are still figuring out exactly how it gets through. Some of it seems to leak through endothelial cells, and a little more might make it into the cerebrospinal fluid and then sneak in through the ventricles. But this isn’t very much, and it can’t reach most of the brain. Instead, the little bit that reaches the brain activates a part of the brain stem called the nucleus tractus solitarii which acts as a sort of relay station, producing its own GLP-1 as a neurotransmitter which it sends to other parts of the brain.
This new neuronal GLP-1 makes it to at least two other brain regions: the arcuate nucleus and the mesolimbic system.
The arcuate nucleus is a pinhead-sized collection of neurons in the hypothalamus. By this point there are dozens of “master regulators” for hunger, but the master master regulator is probably the ventromedial part of this structure. It has two populations: AgRP neurons, (which make you feel hungry) and POMC neurons (which make you feel full).

Each of these populations is downstream of several other slightly-less-masterful master regulators of hunger; one of these is a group called TRH neurons. The TRH neurons use GABA to inhibit the AgRP neurons, thus making you less hungry. And the TRH neurons have GLP-1 receptors. So GLP-1 (and GLP-1 agonist drugs like Ozempic) activate the TRH neurons, which in turn inhibit the AgRP neurons, which in turn makes you less hungry.

We’ll come back to the mesolimbic system later. For now, have this flowchart:
Interlude: Do All Treatments Work Through GLP-1?
The converse of the original question. How many treatments that we thought didn’t involve GLP-1 are actually just GLP-1 drugs in disguise?
How about diet? A thousand diet gurus insist that it’s not just about calorie-counting - some foods help you lose weight faster than others. Why should that be?
Remember, the intestine naturally releases GLP-1 when it detects food, in order to tell the rest of the body to be full. What if some foods make the small intestine release more GLP-1 than others? A diet focused on those foods could be like nature’s Ozempic.
(every diet guru who read that sentence is calling their lawyer to see if they can trademark the phrase “Nature’s Ozempic”)
So we have 9 Foods And Supplements That Increase GLP-1 Naturally, 6 Foods That Increase GLP-1 Levels, and Foods That Naturally Mimic GLP-1. I regret to tell you these are mostly the ones you already knew were healthy - fish, eggs, vegetables, whole grains. There’s also some discussion of supplements including psyllium (plausible) and curcumin (all results related to curcumin are false until proven otherwise). Needless to say, people have already written up arguments for why the GLP-1 evidence supports the paleo diet, the Mediterranean diet, the Ayurvedic diet, the carnivore diet, and the plant-based diet.
How about exercise? Sure, see for example Effects of Exercise On Glucagon-Like Peptide 1, Does Exercise Potentiate The Effect Of [GLP-1] Treatment?, and Endurance Training Improves GLP-1 Sensitivity and Glucose Tolerance in Overweight Women. Mostly this seems to happen by making tissues more sensitive to the effect of GLP-1. Why? Exercise creates very high demand for glucose, so it kind of makes sense that it’s a signal for the body to focus on keeping its glucose system well-tuned.
Whenever we discover a new wonder drug, scientists rush to demonstrate that all those lifestyle changes - the ones you should do anyway - actually work through the same mechanism as the wonder drug. So for example, when SSRIs were the hot new thing, psychiatrists announced that all the normal stuff that brightens your mood worked through serotonin. Sunlight? Serotonin. Exercise? Serotonin. Having a good, trauma-free childhood? Serotonin. In the cold light of day and/or SSRI patents expiring, most of these effects were later found to be fake, or at least too small to care about.
Now that GLP-1 drugs are exciting / on-patent, we’re going through the same process. Everything works through GLP-1! GLP-1 makes the sun shine! GLP-1 makes the grass grow! Nobody knows what caused the Big Bang, but cosmologists are increasingly convinced that GLP-1 might have been involved! You should come back in ten years and check which of these claims have survived. My guess is very few.
For a while, I was taken in by one of these: bariatric surgery, aka gastric bypass. This is a giant mystery. We tell patients that it works by making the stomach smaller so you can’t fit as much food in, but that’s just a tiny part of the effect. We thought that was what would cause weight loss, we invented the surgery on that basis, but surprise! - a bunch of metabolic parameters change before the patient has even had time to lose any weight, and the weight loss tracks these metabolic parameters, not the stomach size. Some scientists thought maybe this was GLP-1 too. After all, the intestine secretes GLP-1 in response to food. The stomach usually digests a lot of food before it even reaches the intestine. But If you remove/shrink the stomach, it can’t do that, and much more food hits the intestine. That means the intestine releases much more GLP-1. And that means the patient feels much more full, much more quickly.
Cool theory, but it turns out gastric bypass works just as well on rats with no functional GLP-1 receptors. Now this is back to being a giant mystery.
Addiction
Speaking of giant mysteries…
It’s not surprising that an intestinal hormone could treat both diabetes and obesity. When you eat a big meal, your body needs to deal with the sudden blood sugar spike. And it also needs to signal to the brain to be full. At least in retrospect, all of this makes sense.
But Ozempic and other GLP-1 drugs appear to be a promising treatment for alcoholism, smoking, stimulant addition, opioid addiction, and maybe even behavioral addictions like shopping. Why?
Let’s go back to the second pathway by which GLP-1 causes weight loss, which goes through the mesolimbic system, aka ventral tegmental area and nucleus accumbens, aka the reward center.
The nucleus tractus solitarii uses neurotransmitter-GLP-1 to inhibit the ventral tegmental area, which then releases less dopamine into the nucleus accumbens. Dopamine levels in the nucleus accumbens act as a multiplier for reward (that is, a given reward feels more rewarding when there’s high dopamine in those areas).
Speculatively, there are two reasons you might eat. First, because you feel hungry. Second, because you crave delicious food. So to fulfill its evolutionary role of making you eat less, GLP-1 needs to do two things. First, it needs to make you less hungry (via the arcuate nucleus / master-hunger-regulator pathway). Second, it needs to make you crave food reward less (via the mesolimbic / reward-center pathway).
Broad-spectrum dampening of the reward system is a terrible fate. Some antipsychotic drugs like haloperidol do this. Take too much haloperidol, and you’ll sit motionless until you die, because no action feels worth it. But the existence of silver bullet anti-addiction medications - Ozempic isn’t the only one, naltrexone seems to treat a whole host of different drug and behavioral addictions - suggests there’s also a sort of narrow-spectrum dampening, one which affects addictions and nothing else.
Why? Isn’t addiction just the extreme version of normal wanting? Apparently not. None of these anti-addictive drugs affect wholesome rewards like the feeling of a job well done or a child’s smile. Just drug addictions, and a few compulsive behaviors like porn and gambling. Maybe the job well-done and the child’s smile get implemented partly through some system other than dopamine (oxytocin?), or maybe these medications lop off some extreme part of the reward distribution that only addictive drugs ever reach in real life. But why? Why did God give your brain a special lever that only porn and cocaine can pull?
GLP-1 suggests maybe this was originally a food reward system. Or at least food was a big enough part of its portfolio that it was a weird but functional hack for a satiety-signaling chemical to just turn off a whole subsection of the reward system. “You’re already full and well-nourished; why would you need the ability to crave things?”
Here’s Skibicka (2013):
Selective and local VTA microinjection of EX4 consistently yields reduction in food intake and body weight. These intake suppressive effects are not macronutrient specific since the intake of both palatable (high-fat or high-sugar) food and normal chow (Alhadeff et al., 2012; Dickson et al., 2012) is reduced by GLP-1R activation. The finding that exogenous stimulation of GLP-1R results in suppression of food intake irrespective of its macronutrient content is perhaps consistent with previous data that indicate that all macronutrients (carbohydrates, fat, proteins) can induce the release of GLP-1 from the intestinal L-cells (Reimann, 2010; Diakogiannaki et al., 2012). Intake of chow, however, is only reduced if the rats are overnight fasted or, in ad libitum fed rats, if the chow is available as the only source of calories (Dickson et al., 2012). In contrast, if a choice between chow and high-fat diet is given to satiated rats EX4 appears to selectively reduce the high-fat intake but surprisingly increase the chow intake (Alhadeff et al., 2012). These findings can lead us to conclude that VTA GLP-1R activation might result in a lack of preference for high-energy/fat food.
If I’m understanding this right, the researchers found two separate behavioral effects of GLP-1. First, it made rats eat less. But second, when presented with very tasty food vs. normal food, it made the rats stop preferring the very tasty food. At least in this study, the extreme part of the reward system that governs addiction got used to determine how much to prefer very tasty food over normal food2.
If you want to learn more about GLP-1 receptor agonists and addiction, including the application to public policy, I highly recommend the Recursive Adaptation blog.
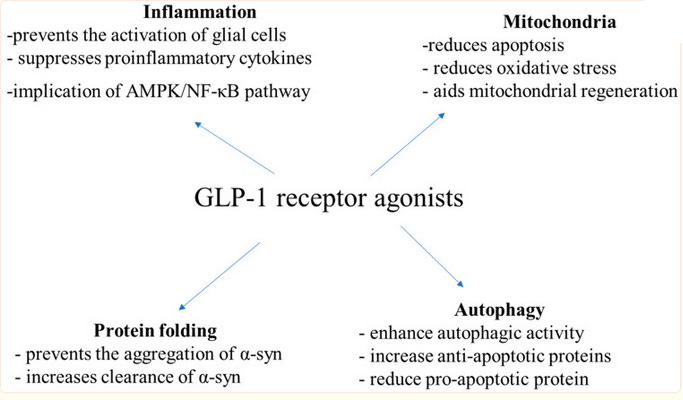
Alzheimers And Parkinson’s
Okay, now God is just trolling us.
GLP-1 Receptor Agonists: A New Treatment in Parkinson’s Disease gives us this diagram:
…which, as is a running theme in this post, doesn’t make me feel more enlightened in any way. Why does GLP-1 do all these things?
Diabetes is a well-known risk factor for Parkinson’s, Alzheimers, and other dementias. The exact mechanism isn’t clear, but the extra sugar that diabetics have in their blood tends toward uncontrolled reactions with various other chemicals, creating slightly toxic glycosylated species that damage the cells. So the simplest mechanism by which GLP-1 drugs could prevent dementia is by lowering the concentration of these toxic metabolites.
But GLP-1 drugs also prevent dementia in non-diabetics, so there has to be more going on.
Most of the relevant papers say the drugs work by preventing inflammation. This is a catch-all term for the immune response to microbes; although it helps fight the microbes, it’s slightly toxic to the rest of the body and generally bad unless you’re actively fighting an infection. In chronic inflammation, ie the thing most of us with modern diets have all the time, general bad health damages the body, the immune system mistakes the damage for a microbial infection, and it provokes a constant low-grade inflammatory response. This is bad, so (if you’re not fighting an infection) anti-inflammatories are generally pretty useful. There are lots of anti-inflammatory drugs (aspirin is one, ibuprofen is another), but inflammation is a multifaceted process and no one drug can stop it entirely.
GLP-1 drugs seem to be especially potent anti-inflammatories that stop some of the inflammatory processes most implicated in dementia.
How? Research is still very early, but the best explanation I can find is in Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. This team found that there are no GLP-1 receptors on immune cells, so these drugs can’t be affecting the immune system directly. The researchers hypothesized that the drugs must be affecting the parts of the brain that regulate the immune system, especially the back of the brain stem. So they injected some of the drugs directly in to the brain, and sure enough, this was enough to produce the effect. How do the brain cells communicate with the immune cells? The team tediously injected one of each kind of chemical that blocks each kind of chemical communication system, and found that only the alpha-blockers and the delta-opioid blockers prevented GLP-1’s anti-inflammatory effects. So probably GLP-1 binds to neurons in the brain stem, those signal to other neurons and immune cells via alpha-adrenergic receptors and delta-opioid receptors, and then the immune cells initiate an inflammatory reaction.
Same question as before: why would an appetite-related hormone do this? I can’t find anyone studying this question, so I asked Claude. It had some surprisingly clever guesses. Food itself causes a mild inflammatory response (because it usually contains suspicious-looking foreign chemicals); insofar as this is a common false alarm, the body might want to suppress immune response when it knows food is coming. But also, when a meal comes in, the body diverts other resources towards the digestion process (this is why a big lunch makes you tired). Maybe some of those resources come from the immune system, so immune cells stand down while you’re digesting.
That’s as far as I got with this one, but this is a very active area of research and we’ll hopefully know more soon.
And Many More…?
Just this month, a study found GLP-1 drugs cut risk of some obesity-associated cancers. I haven’t had a chance to read it yet, but I expect this to be a theme of the next few years - more new exciting GLP-1 effects than we can keep up with. Along with all the specific mechanisms above, there’s a more general question: what do we think when we keep finding new indications for these drugs?
Modern Westerners eat too much food. This is bad in various ways. So if GLP-1 drugs reduce obesity, that has the potential to be good in various ways. This makes sense and is definitely part of the story. But some of these effects (eg addiction) aren’t obviously linked to obesity. And others that seem linked to obesity (eg heart disease) turn out to be obesity-independent; scientists can observe them even in weight-neutral rats. So this doesn’t explain everything on its own.
Some of those explanations will be evolutionary. GLP-1 is a master signal for the starving vs. well-fed state. Lots of bodily processes change based on whether you’re starving vs. well-fed. Naively you’d expect that there would be as many side effects as positive effects (wouldn’t some conditions be better in the starving state?), but maybe that’s not true. In particular, maybe the inflammatory nature of the starving state really hurts a lot of systems. Maybe we’ll manage to trace everything back to various food-related pathways, until all of GLP-1’s effects feel natural and satisfying.
Other explanations will be more sinister. Pharma companies are always looking for more reasons to prescribe their drugs. And even unaffiliated scientists can get caught up in the excitement. Being a GLP-1 researcher now is probably a pretty great job, just like being an SSRI researcher thirty years ago. Probably some of these won’t replicate, and in a few years we’ll be left with a thinner and more believable profile of GLP-1 effects.
But I don’t believe it’s all pharma marketing. These drugs seem really special. We’re going to have an exciting next few years as we fully unwrap this weird new gift Nature has given us.
Thanks to Stephan Guyenet, who reviewed an earlier draft of this post. As always, any mistakes are mine alone.
Sex is the other extremely-rewarding, sometimes-addictive behavior old enough that it might have shaped the evolution of the reward system. Do GLP-1 receptor agonists lower libido? Mouse studies say maybe, but human studies say no. A very straightforward extrapolation of the Skibicka result might suggest that GLP-1 drugs shouldn’t change libido overall, but should make people less intensely prefer good sex to bad sex. So far nobody has managed to get that study premise past an IRB, sorry.









Share this post